Crazy as it might seem, there was a time when the U.S., the Soviet Union, and other countries testednuclear weaponsby exploding them in the atmosphere. From 1945 to 1963, when such tests finally werebanned by an international treaty, more than 500 nuclear bombs were detonated, releasingradioactive falloutthat spread far and wide across the planet, causing harm to the environment and human health.
For example, everyone born in the U.S. after 1951 has been exposed to nuclear fallout, and for some, it's resulted in an increased risk of thyroid cancer, according to theU.S. Centers for Disease Control and Prevention(CDC).
Advertisement
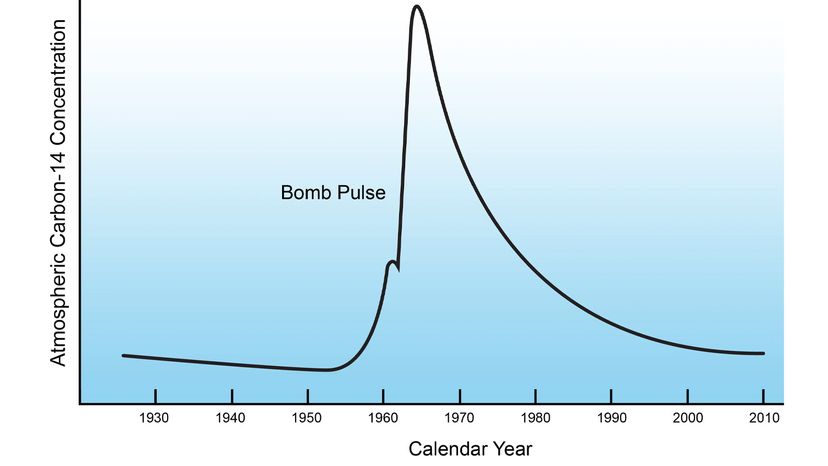
But for scientists that fallout also has provided an important measuring tool. The tests caused what's known as the14C bomb pulsebecause of thespike in the atmospheric concentrationof carbon-14, a carbon isotope that also occurs naturally. The excess carbon-14 was distributed throughout Earth's atmosphere, peaking in 1963 when the test ban went into effect.
That radioactivity, which has gradually been declining since the 1960s, has been absorbed by plants, animals and people, creating a sort of time stamp that's enabled researchers to measure when things occur — from thelongevity of white sharksto thegrowth of human knee cartilageand evenbrain cells. It's also enabled forensic investigators to estimate the age and year of death for human remains with much greater precision than previously possible.
Advertisement